ELECTRONIC CONFIGURATION: Arrangement of Electrons into shells for an Atom (E.g Electronic Configuration of Carbon is 2 . 4 )
sELECTRONIC CONFIGURATION AND POSITION IN PERIODIC TABLE
s- Number of notations in electronic configuration will show the number of shells of Electrons the Atom has, showing the Period
- Last Notation shows the number ofouter Electrons the Atom has, showing the Group
EXAMPLE: Electronic Configuration of Chlorine:
PERIOD: Red numbers at the Bottom Show the number of notations which is 3, indicating that Chlorine atom has 3 shells of Electrons
GROUP: Green box highlights the last notation which is 7, indicating that Chlorine atom has 7 outer Electrons
ON THE PERIODIC TABLE: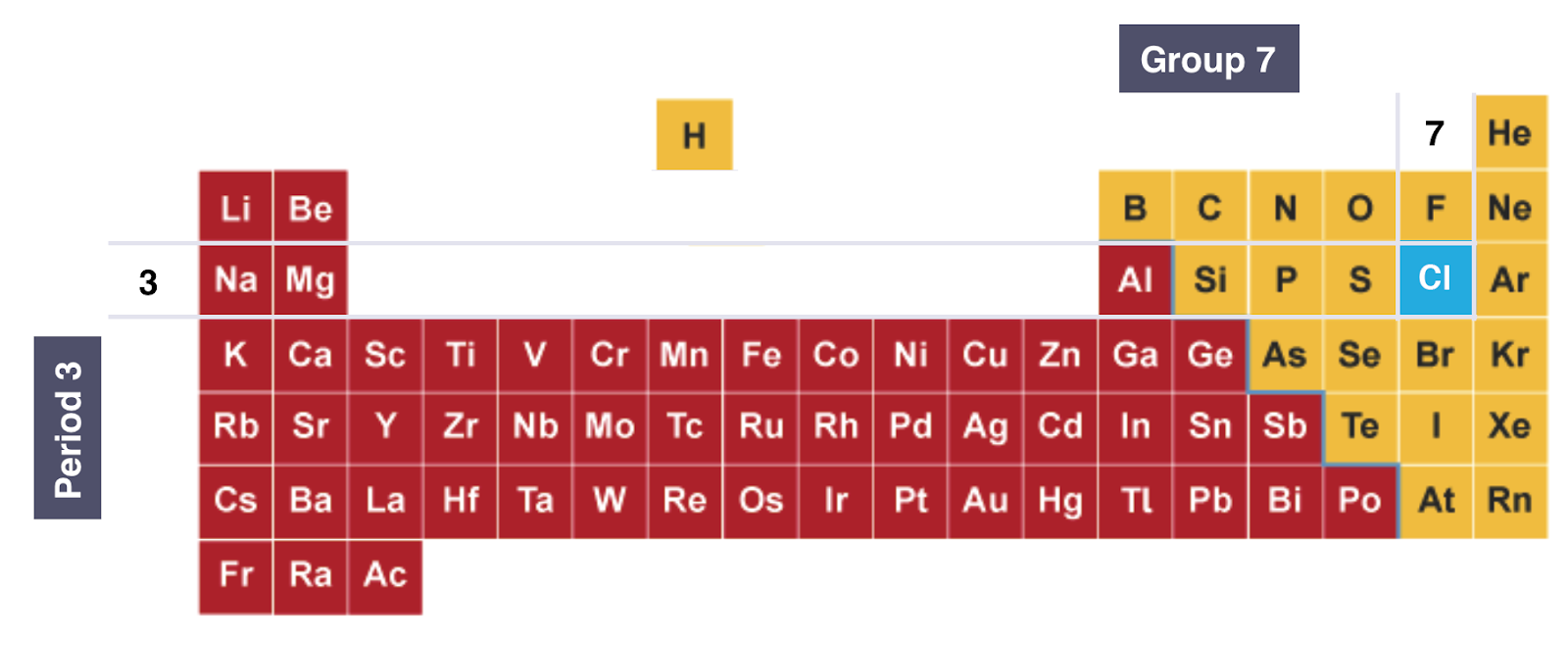
Diagram showing the position of Chlorine on the Periodic Table
No comments:
Post a Comment